Categories
Categories
Brands
Brands
- Home
- Interventional Cardiology / Radiology / Electrophy
- Abbott
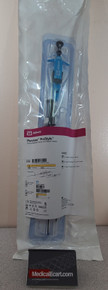
- Abbott 12673-03 Perclose ProGlide ® Suture-Mediated Closure System. Box of 10
Abbott 12673-03 Perclose ProGlide ® Suture-Mediated Closure System. Box of 10
Product Description
Abbott 12673-03 Perclose ProGlide ® Suture-Mediated Closure System. Box of 10
(1) Perclose ProGlide ® Suture-Mediated Closure Device (1) Suture Trimmer (1) Snared Knot Pusher Box of 10
INCLUDES :
(1) Perclose ProGlide® Suture-Mediated Closure Device
(1) Suture Trimmer
(1) Snared Knot Pusher
INDICATIONS
The Perclose ProGlide SMC System is indicated for the percutaneous delivery of suture for closing the common femoral artery and vein access site of patients who have undergone diagnostic or interventional catheterization procedures.
The Perclose ProGlide SMC System is used without or, if required, with adjunctive manual compression.
For access sites in the common femoral artery using 5F to 21F sheaths.
For access sites in the common femoral vein using 5F to 24F sheaths.
For arterial and venous sheath sizes greater than 8F, at least two devices and the pre-close technique are required.
CAUTION
Federal law restricts this device to sale by or on the order of a physician (or allied healthcare professionals, authorized by, or under the direction of, such physicians) who is trained in diagnostic and / or interventional catheterization procedures and who has been trained by an authorized representative of Abbott Vascular.
Prior to use, the operator must review the Instructions for Use and be familiar with the deployment techniques associated with the use of this device
During closure of access sites using a procedural sheath greater than 8F it is recommended that a vascular surgeon or a surgeon with vascular training be available in case surgical conversion to control bleeding and to close the vessel is needed.
CONTRAINDICATIONS
There are no known contraindications to the use of this device. Attention is drawn to the WARNINGS and PRECAUTIONS sections.
 Loading... Please wait...
Loading... Please wait...