Categories
Categories
Brands
Brands
- Home
- Interventional Cardiology / Radiology / Electrophy
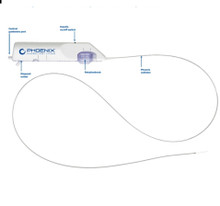
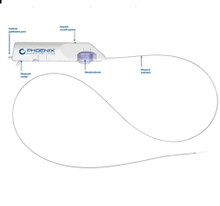
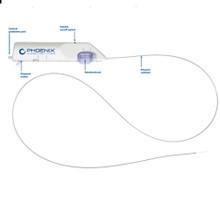
- PD22130K Phoenix Atherectomy System, Catheter size 2.2mm x 130cm deflecting, Introducer size 6F (>2.2 mm), Working length 130cm , Guidewire diameter 0.14". Box of 01 Kit
- Home
- Interventional Cardiology / Radiology / Electrophy
- Volcano
- PD22130K Phoenix Atherectomy System, Catheter size 2.2mm x 130cm deflecting, Introducer size 6F (>2.2 mm), Working length 130cm , Guidewire diameter 0.14". Box of 01 Kit
PD22130K Phoenix Atherectomy System, Catheter size 2.2mm x 130cm deflecting, Introducer size 6F (>2.2 mm), Working length 130cm , Guidewire diameter 0.14". Box of 01 Kit
$5,999.00
PD22130K Phoenix Atherectomy System, Catheter size 2.2mm x 130cm deflecting, Introducer size 6F (>2.2 mm), Working length 130cm , Guidewire diameter 0.14". Box of 01 Kit
$5,999.00
Product Description
PD22130K Phoenix Atherectomy System, Catheter size 2.2mm x 130cm deflecting, Introducer size 6F (>2.2 mm), Working length 130cm , Guidewire diameter 0.14". Box of 01 Kit
The Phoenix rotational atherectomy system combines the benefits of existing atherectomy systems to deliver a unique atherectomy option. This will help you tailor your treatment approach for your patients.
SAFE
| Clinical concern | Phoenix solution | Safety data |
| Vessel injury | Front cutter clears tissue in a way that may help reduce potential trauma to the vessel | 1.9% perforation, 0.9% dissection |
| Distal embolization requiring intervention | Design of the Phoenix cutter head allows debulked material to be continuously captured | <1% distal embolization 0% use of distal protection |
Effective
- EASE trial data confirms Phoenix’s ability to effectively treat a broad range of tissue types, from soft plaque to calcified arteries, for lesions both above and below the knee.
- The effectiveness endpoint set in the EASE trial was exceeded, <1% clinically driven target lesion revascularization (TLR) was achieved>and a <1% clinically driven target lesion revascularization (TLR) was achieved
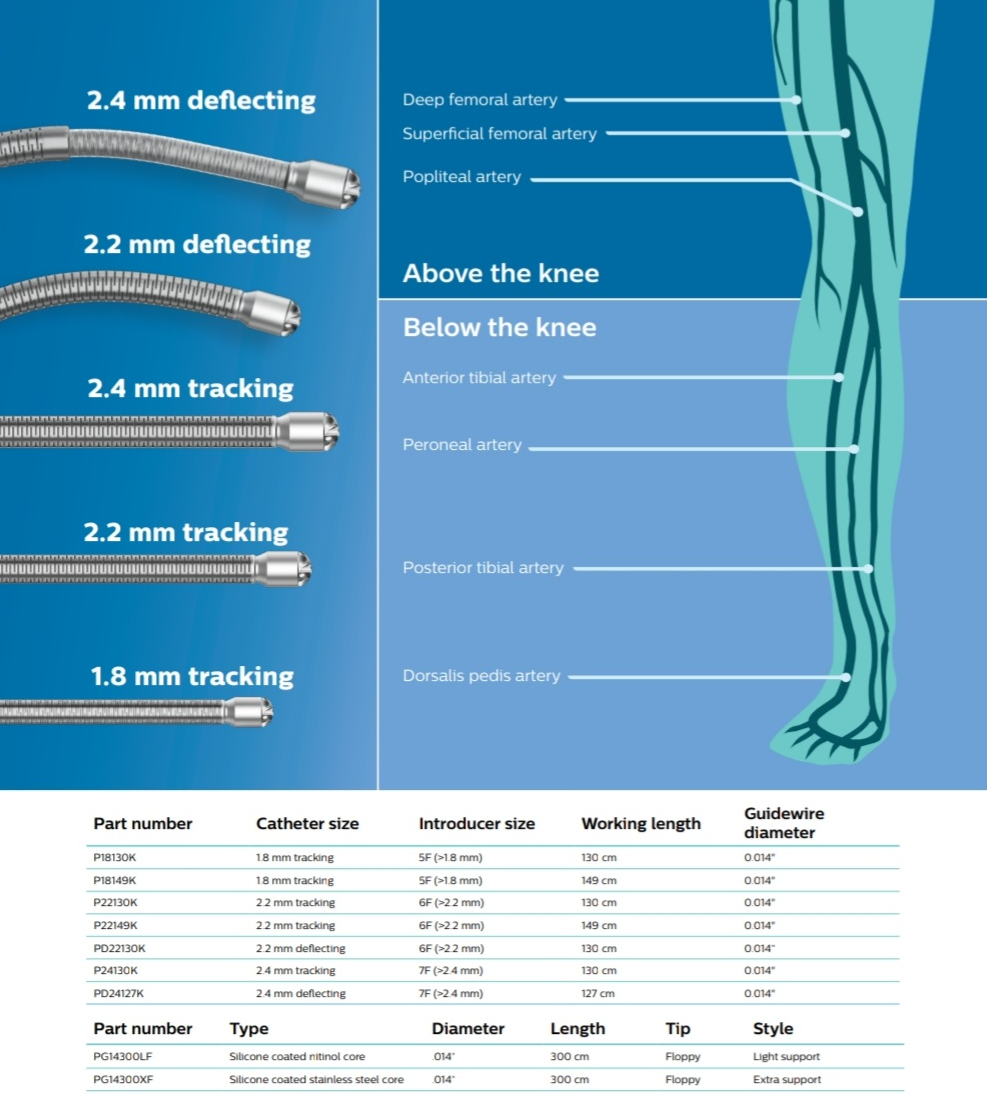
- Three catheter diameters have been shown to effectively treat most peripheral vasculature.2 - 1.8 and 2.2 mm (tracking) are suited for treating small vessels or highly stenosed lesions. - 2.4 mm (deflecting) is suited for larger vessels or eccentric lesions.
Easy
- Single insertion: no need to remove and clean out debulked material.
- Battery powered handle operated. No capital equipment or additional procedural accessories required.
- Low profile, front cutting design allows for direct lesion access.
 Loading... Please wait...
Loading... Please wait...